br electron configuration|7.4: Electron Configurations of Ions : Bacolod Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa Conform informațiilor afișate pe site-ul www.unibet.ro, aceste metode de contact Unibet sunt disponibile zilnic în intervalul orar 09:00 – 24:00, ceea ce este un mare avantaj și practic vei primi ajutor aproape în orice moment al zilei. În plus, în afara acestui interval de timp poți apela la chat-ul live în limba engleză, care este non-stop și poți fi ajutat și .
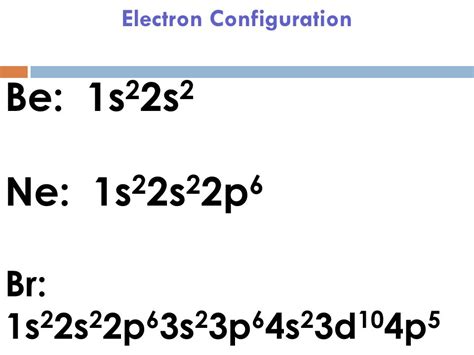
br electron configuration,Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of bromine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5. In the bromine ground-state electron configuration, the last five electrons of the 4p orbital are located in the . Tingnan ang higit paThe total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit paThe electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons . Tingnan ang higit paHun 23, 2019 — Learn how to write the electron configuration for Br-, the Bromide ion, and why it is the same as Argon. See examples, explanations and diagrams of orbital filling and ion formation.The electronic configuration for $\ce{Br-}$ is: $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ Because it have one more electron than bromine, which ends its electronic .
br electron configuration 7.4: Electron Configurations of Ions 119 rows — Mar 23, 2023 — Find the electron configuration of boron (B) and .Peb 1, 2021 — Beryllium Electron Configuration. Bromine Orbital Diagram. Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is from bottom to top, that .Abr 9, 2024 — Aufbau principle. First, find electrons of bromine atom. Periodic table | Image: Learnool. The atomic number of bromine represents the total number of electrons of bromine. Since the atomic number of bromine is 35, .br electron configurationLearn how to determine the electron configuration of cations and anions using Aufbau's principle, Pauli-exclusion principle, and Hund's rule. Understand the magnetic properties of .Nob 18, 2022 — The electronic configuration notation of Br is represented by the notation; Br : Ar18 4s2 3d10 4p5. Bromine unabbreviated electron configuration. Br: 1s2 2s2 2p6 3s2 3p6 4s2 .Nob 13, 2020 — Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron Configuration. The periodic table is a tabular display of the chemical .By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram for a selection of .Nob 13, 2020 — Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure.The chemical symbol for Bromine is Br. Electron Configuration and Oxidation States of Bromine. Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron ConfigurationThe first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers and electron .Peb 28, 2018 — The electron configuration of Bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[Ar] 4s^2 3d^10 4p^5#. Explanation: Use a chart such as the one below to fill the subshells in order of the diagonal lines.
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .Hun 20, 2023 — The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the .
Peb 1, 2021 — An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled whenever possible. . Br: 2: 2 6: 2 6 10: 2 5 .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each orbital can hold two electrons, one .Bromine(Br): Bromine is a p-block element having an atomic number 35. Bromine belongs to group 17 which is known as halogens. Electronic configuration of Group 17: The elements of group 17 have seven electrons in their outermost shell. So, the valence electronic configuration of group 17 is n s 2 n p 5. Electronic configuration of Bromine:The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.Reduced electronic configuration Br: [Ar] 3d 10 4s 2 4p 5. Below is the electronic diagram of the Bromine atom Distribution of electrons over energy levels in the Br atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 18 4-st level (N): 7. Valence electrons of Bromine.
Bromine (Br) element properties, information, facts, uses and Periodic Table trends. Complete information about the Bromine element - Atomic Number 35, atomic mass [79.904], melting point, How to Locate on Periodic Table, History, .
Nob 18, 2022 — Bromine unabbreviated electron configuration. Br: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. When there is no noble gas configuration for the starting electrons, this is referred to as an unabbreviated electronic configuration. ground state Bromine electron configuration. The ground state electronic configuration of Br will be 1s 2 2s 2 2p 6 3s .Hul 20, 2022 — Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s 2 (spoken as “one-ess-two”). Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; and f, 14 (Table .
Dis 24, 2015 — What is the electron configuration for bromine? Chemistry Electron Configuration Electron Configuration. 1 Answer Zach Dec 24, 2015 [Ar] #4s^(2)3d^(10)4p^5# Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full .7.4: Electron Configurations of Ions The electron configuration of an atom describes the orbitals occupied by electrons on the atom. The basis of this prediction is a rule known as the aufbau principle, . Br [Ar] 4s 2 3d 10 4p 5: 36: Kr [Ar] 4s 2 3d 10 4p 6 = [Kr] Exceptions to Predicted Electron Configurations .

Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 .Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens . (2.55), the carbon atom in a C–Br bond is electron-deficient and thus electrophilic. The reactivity of organobromine compounds resembles but is intermediate between the .
br electron configuration|7.4: Electron Configurations of Ions
PH0 · How can I figure out the electron configuration of Br
PH1 · Electron Configuration Chart of All Elements (Full Chart)
PH2 · Complete Electron Configuration for Bromine (Br, Br
PH3 · Bromine electron configuration
PH4 · Bromine Electron Configuration :7 Easy Steps on How to Write
PH5 · Bromine Electron Configuration (Br) with Orbital Diagram
PH6 · Bromine
PH7 · Br
PH8 · 7.4: Electron Configurations of Ions
PH9 · 2.6: Electron Configurations